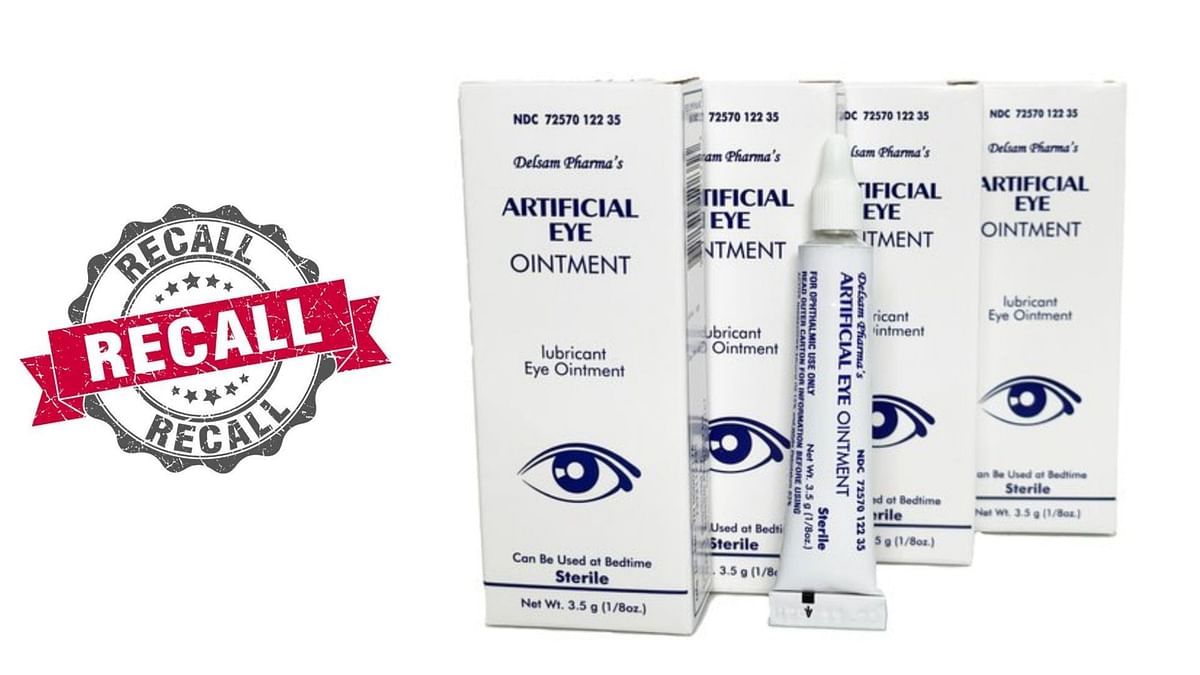
Artificial Tears Reca For Eye Irritation 2025 Schedule. The products included in the recall were shipped between may 26, 2023, and april 21, 2025. When were the items distributed?
.jpg)
The recalled products were shipped from may 26, 2023, to april 21, 2025, avkare said. The recalls involve artificial tear eye drops, gels, and ointments. Artificial tears ophthalmic solution, dextran 70.01%/glycerin 0.2%/hypromellose 0.3% (eye lubricants) lubricant eye drops, sterile,.


.jpg)



.jpg)


